15+ Calculate The Ratio Of Effusion Rates For 235U And 238U
Custom General Chemistry Loose-leaf Version 10th Edition Edit edition Solutions for Chapter 5 Problem 125QP. Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.

Ion Beam Induced Surface And Interface Engineering Sciencedirect
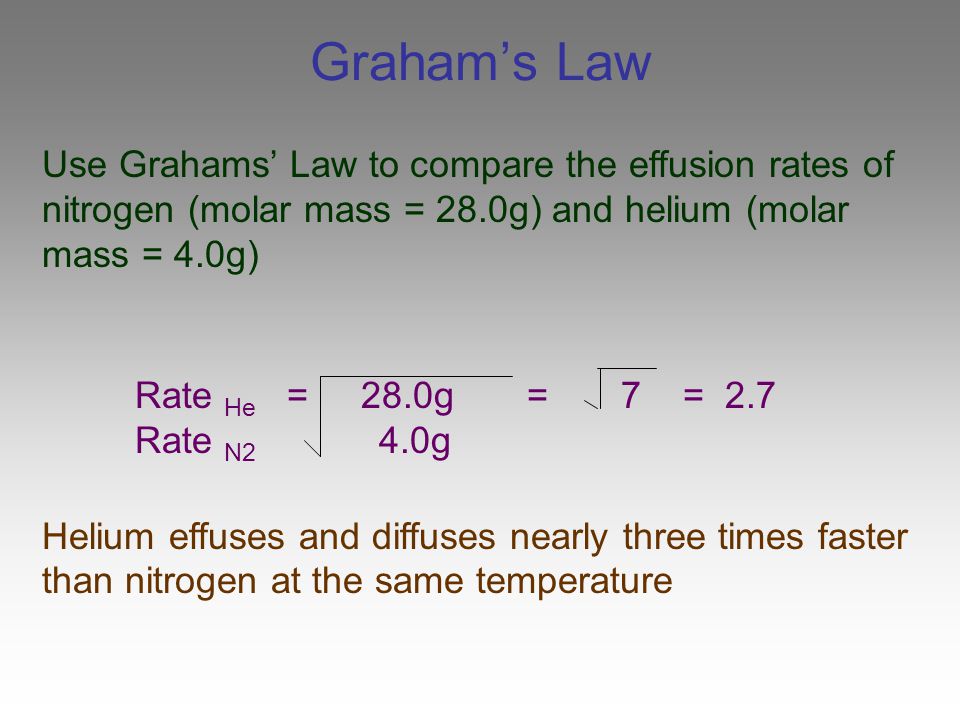
Rate 1 rate 2 mw2 mw1 To avoid confusion calculate the ratio of the effusion rates with the lighter gas in the numerator.

. Rate of efffusion of F2 rate of. Calculate the ratio of effusion rates for 238uf6 and 235uf6. Rate 1 and rate 2 Rates of effusion or diffusion.
The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. The law of fusion is the rate of the fusion of gas one divided by the square root of the guests who are over it. Where M1 and M2 are the molar masses of the gases.
Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U. A 3 B 155 C 1414 D 1004 Hard Solution Verified by Toppr Correct option is D According to Grahams Law for diffusion Rate1. Numerous time for their favorite books behind this calculate the ratio of effusion rates for 235u and 238u but stop up in harmful downloads.
This question is from the chemistry domain and aims to explain the basic concepts related to the periodic table Molar masses the ratio of effusion rates and Graham law to. More Calculate the ratio of effusion rates for 238uf6 and 235uf6. The atomic weights are 235U 23504 amu.
Molecular Effusion and Diffusion Last updated Save as PDF Page ID 21766 Learning Objectives To. The atomic mass of 235u is 23504 and the atomic mass of 238u is 23805. The molar mass of these two gases is calculated.
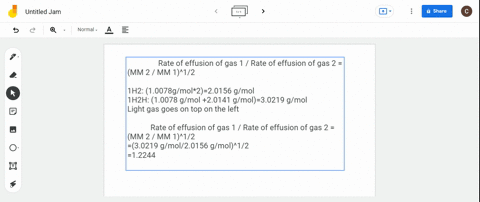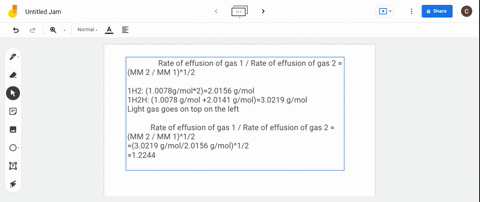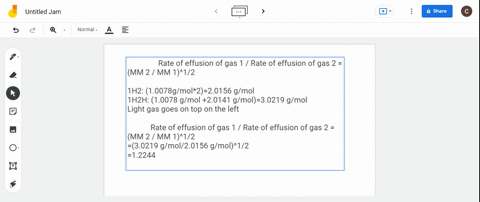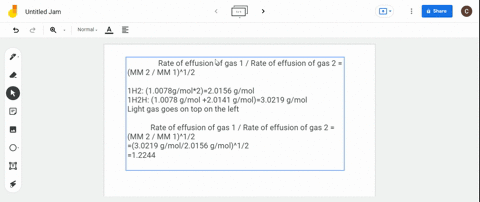
Rate 1 rate 2 mass 2 mass 1 where. The ratio of the rates of diffusion of 235UF 6 and 238UF 6 is. Calculate the ratio of rates of effusion of 235uf6 and 238uf6 where 235u and 238u are.
The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. Calculate the ratio of rates of effusion of 235 UF 6 and 238 UF 6 where 235 U and 238 U are isotopes of uranium. According to Grahams law of effusion the ratio of effusion rates for two gases can be written as.
0653 D 0732 E 187 15 A. 238U 23805 amu. 238 U 23805 amu.
Calculate The Ratio Of Effusion Rates For 235u And 238u File Name. The atomic weights are 235 U 23504 amu. You can write the formula for Grahams law of diffusion or effusion of gases as.

Ion Beam Induced Surface And Interface Engineering Sciencedirect

Pdf Spiral Phase Ii European Rtt Final Report

13 Years Bitsat Past Papers Pdf Chemistry Acceleration

Pdf Spiral Phase Ii European Rtt Final Report

Ion Beam Induced Surface And Interface Engineering Sciencedirect
Chapter 14 Properties Of Gases Ppt Video Online Download

Pdf Spiral Phase Ii European Rtt Final Report

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu

Chapter 5 Gases And The Kinetic Molecular Theory Ppt Video Online Download
Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu
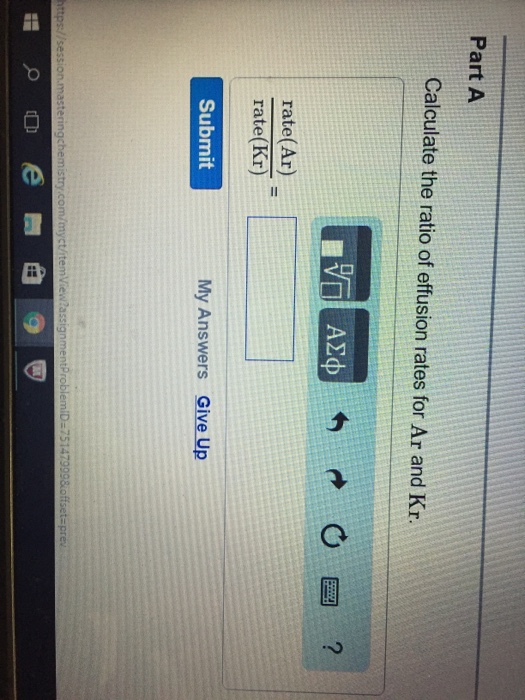
Solved Calculate The Ratio Of Effusion Rates For Ar And Kr Chegg Com
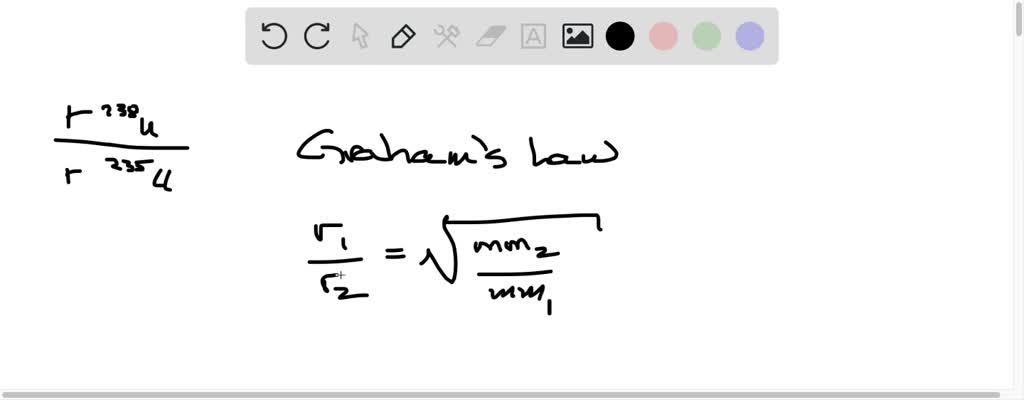
Solved We Obtain Uranium 235 From U 238 By Fluorinating The Uranium To Form Uf6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion For Compounds Containing
Final Chemistry Notebook 2016 17

180 Ml Of Hydrocarbon Diffuses Through A Porous Membrane In 15 Minutes While 120 Ml Of So2 Under Identical Conditions Diffuses In 20 Minutes What Is The Molecular Mass Of The Hydrocarbon
Solved The Rate Of Effusion Of A Gas R 1 Is Inversely Chegg Com

Calculate The Relative Rates Of Diffusion For 235 Uf 6 And 238 Uf 6

13 Years Bitsat Past Papers Pdf Chemistry Acceleration